CS 39J: Session 7
http://inst.eecs.berkeley.edu/~cs39j/session07.html
7 March 2002
OPTICAL video
Prof. Barsky shows both the "voice-over" and "lite" versions
of "Post-Processing Sharp Images for Optically Correct Blur", the
research video that he and his OPTICAL group have just finished for submission
to the SIGGRAPH Computer Animation Festival. Comparing the two versions,
the students in class seem to prefer the voice-over version.
Dr. John Below's introduction
Dr. John Below, a photographer who is an expert in chemistry, is guest-lecturing
today.
Dr. Below teaches at the UC Berkeley Extension, lecturing about the scientific
basis of photography.
Comparison of digital and "silver" (or classic) film
| |
Ag (silver halide) |
digital |
| cost |
initially cheaper |
cheaper in the long run |
| sharpness |
+ sharper |
improving |
| flexibility |
|
+ |
| ease & speed |
|
+ |
Both are similar in many respects, particularly in the mechanics they have.
How film works
Digital "film" uses an array of small semiconductor device sensors
called pixels that "see" a portion of the screen and send an electrical
signal to the amplifiers, electronics, and to the rest of the computer system.
Today's silver halide film has an emulsion, which is cast over transparent plastic.
This emulsion is comprised of minute crystals of silver compound suspended in
gelatin, which are made of one or more of these compounds: silver bromide (AgBr),
silver chloride (AgCl),which was once used for contact paper, and silver iodide
(AgI). Most film is made from AgBr and AgI. These crystal are formed by mixing
silver nitrate with a soluble salt of bromide or iodide in a water solution
containing gelatin:
AgNO3 + NaBr —> NaBr + NaNO3
The resulting silver compound is very insoluble and precipitates out as fine
crystals.
The emulsion consists of ions, not atoms, stuck together. The crystals are
analogous to pixels.
Let's digress slightly to the history of scientific understanding of light.
There is the wave theory of light and the particle theory of light. Sir Isaac
Newton treated light like particles, that is, bullets through the air. Other
scientists favored modeling light as waves. In fact, light has a "wave-particle
duality" meaning that it exhibits both wave-like and particle properties.
|
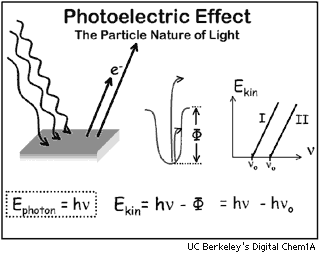
Albert Einstein, in 1905, announced the Photoelectric Effect, for which
he subsequently received a Nobel Prize. He noted that light shining on
certain metals like cesium (Cs) will knock some electrons from that metal.
Einstein said that light is not only a wave, but also a particle. These
particles, called photons, are related to the electrons they produce by
the equation:
E = h * n (Planck's constant)
where:
E= energy of the electron being produced, in ergs
h = 6.626176 x 10-27 ergs Hz-1, Planck's constant
n = frequency in Hz
|
 |
 |
|
|
 |
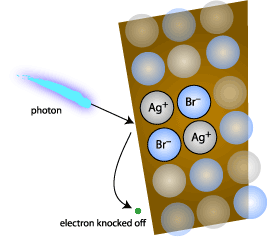
What happens if you shine light on a silver halide such as AgBr? Each
photon of light will knock out an electron from Br-, leaving
a Br atom , which in most cases reacts with the gelatin in the emulsion
leaving an unbalanced Ag+ ion behind. Depending upon the exposure
a number of photons will so behave in each crystal, which was exposed
to light.
Each released electron will drift through the crystals until it
encounters a silver ion where it reacts to form a silver atom.
All these silver atoms taken together are called the latent image, but
are too few and small in size to be seen. With
massive exposures on the order of one or more hours this image could
appear. Early photography actually used these
long exposure times, but it was not practical until the process called
“development” was discovered.
|
Development
Development overcame the problem of requiring a long exposure for an image.
All it takes is having one, two, or three silver particles per grain, which
then catalyze a reaction between the nearby silver ions and a developing agent
(a reducing agent):
There are a class of reducing agents (nowadays 30 or 40 of them, most of
which are organic compounds) which will
reduce silver that has been exposed, and leave the unexposed silver
unchanged.
The image is based on the grains of pure silver metal. The exposed
grains get converted to pure silver (Ag) while the
unexposed grains do not become silver. Some parts of the film that
aren't supposed to get any exposure at all will still
get converted to silver. This is referred to as "fog". For example,
developing a completely unexposed sheet of film will
produce a light, uniform, gray result
Using this treatment we convert these cube-like crystals into
irregularly shaped grains of silver.
Note that longer development times tend to increase contrast and
increase graininess of the images.
When there is no light, very few grains develop, but there is still some fog.
Fixation
This is good old-fashioned chemistry where you add water, plus another compound
X, and it dissolves the silver bromide: X + AgBr(solid) —H2O—>
AgBr(aqueous)
This compound X is Na2S2O3 (sodium thiosulfate). This forms a soluble complex
with the silver ions which increases the solubility of AgBr. This leaves opaque
silver where the light hits and clear space where there was no light.
What about intermediate shades of gray? These exposed grains are
smaller and do not contain as much silver; hence,
they allow some light to come through.
The whole explanation of how the latent image is produced by light is quite
complex; the best description we have is called the Gurney-Mott
theory. This was developed about 50 years ago.
Question Break
Q: You said the amount of silver in a little cube is how dark the image
is. Does this apply to only black & white
photographs?
JB: Color does the same thing, except the manufacturers of the film
couple a dye ("dye coupling") with a silver atom.
When a particular color activates the silver, it also activates the dye.
The silver is later bleached out leaving the dye to
form the image.
Q: Has anyone ever thought that maybe sound travels in particles as well
as waves, also? Has that ever been tested?
JB: The current view is that every material substance has waves associated
with it. Sound is the motion of a plurality of particles. The wave-particle
duality is now thought to exist in all matter. There is a wave associated with
any material object, which is called the DeBroglie wavelength, named after the
French physicist Louis de Broglie.
At 5:05pm, lights go out in Soda Hall! (Why?)
Coming Attractions
H&D Curve (Hurter and Driffield characteristic curve)
chain rule for calculating overall resolving power for the picture
how to intelligently maximize the depth of field without giving away the virtues
of your lens. Stopping down actually makes your lens fuzzier.
John Below completes his lecture during session
11.
Assignment
Take a photograph of some detail someplace. For example, take a close-up
photograph of someone's eye. Be creative and do something interesting :-)
Class is dismissed half an hour early due to the power failure.
Return to the Schedule Page
  |
Current webmaster: Steven Chan ().
CS 39J: The Art and Science of Photography is a freshman seminar taught
by .
Site design by .
|



